The Bioengineering Lab has been working hard over the years to bring studies that really help the scientific community development. The research work in our laboratory has, as main axis, two topics, which are:
Cerebral Autoregulation
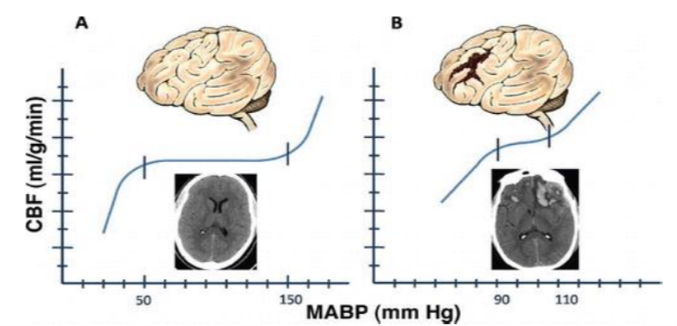
Although the human brain demands a high amount of energy, our brain has a limited supply of it, which makes the organ extremely dependent of a continuous oxygen and energetic substrates through a cerebral blood flow (CBF). Thereby, the brain needs mechanisms that maintain the CBF proper, ensuring it integrity and functionality. One of these mechanisms is the cerebral autoregulation (CA), it protects the brain by keeping the CBF constant between a arterial blood pressure’s (ABP) variation of a range the 60-140 mmHg (Image 1).
Over this fluctuation, the CA changes the diameter of the blood vessels making it increase the size when the ABP is higher or decrease when it is lower. As a result, the CA provides a protection to the brain once it avoids, for example, the cerebral ischemia during hypotension or even excessive blood flow during arterial hypertension which can cause hemorrhagic strokes. In the Bioengineering Lab, we research about the AC mechanism which can be measure through complex techniques by using mathetical methods and signal processing tools as well as Transfer Function Analysis (TFA) and Autoregulation Index (ARI). In that way, we are able to investigate, in different neurological diseases, the status of CA’s subjects to better understanding of the brain pathophysiological mechanisms.
Cardiac Electrophysiology
Several arrhythmias are encountered in clinical practice being atrial fibrillation (AF) the most common. Our understanding of AF mechanism remains quite poor. It is known that the uncoordinated atria electrical activity leads to different pathophysiological processes, including mechanical deterioration. Its sustained long-term presence (persAF) promotes pronounced electrical-contractile-structural atrial substrate remodeling where multiple distinct mechanisms coexist simultaneously or at different times, making critical the decision where to ablate. Understanding the AF behavior is a key factor to contribute towards improving persAF patient outcome. Our research interests focus at developing advanced and customized tools allowing the understanding of wave propagation and mechanisms of this complex arrhythmia, using realistic computational models, animal’s experiments and clinical data. Improving current knowledge of AF throughout individual mapping and “customized” ablation strategy might improve patients’ quality of life and reduce consumption of health resources.
Current projects include:
- Dynamic of foci and reentrant propagation
- Mechanism of atrial fibrillation
- 3D non-invasive and invasive oriented customized cardiac mapping
- Biophysical arrhythmic mechanisms modelling
